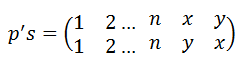Lorentz Length Contraction
(The mathematics of this proof requires the equations that we came up with in the proof of time dilation.)
If we take a an object with a length L0 and move it with a constant velocity 'v' to the right, the object will have a length of L'.
Under Newtonian mechanics (where there would be no time dilation) we can express the x and x' values like this:
However, due to relativity, we must take into account the relativistic time dilation with velocity. This gives us:
The x values can be used to express L0 , and the x' values can be used to express the L' values:
We can then substitute the relativistic values of x1 and x2 into the equation of L0 :
Substituting L' for the numerator gives:
Which can be re-arranged to give:
Therefore, velocity has the effect of contracting the length of a body perpendicular to the direction of motionrelative to the observer.
QED
So if the rest frame were a 3m long spaceship, and it were to blast off at 0.8c relative to an observer it would appear to have the length of 1.8m. As with time dilation, this effect is only noticeable at velocities a sizeable fraction of light-speed.